Ji-Geng Yan & Mary P. Eldridge & William W. Dzwierzynski & Yu Hui Yan & Safwan Jaradeh & Lin-Ling Zhang & James R. Sanger & Hani S. Matloub
Abstract
Compound muscle action potentials (CMAPs) can be used to analyze injury and recovery of nerve. This standardized study evaluates the value of CMAP analysis in predicting the long-term efficacy of neurolysis. CMAP amplitude is also used to determine the optimal extent of neurolysis. The left peroneal nerves of 30 rats were crushed. CMAPs were recorded for both crushed (left) and control (right) nerves. Fifteen rats underwent neurolysis 3 months post crush injury; the remaining 15 were sham controls and did not undergo neurolysis. CMAP measurements were taken after: (1) release of the nerve from the fascia, (2) opening the epineurium, and (3) opening the perineurium. At 3 months post crush injury, opening the epineurium resulted in a statistically significant increase in CMAP. CMAP increase with perineurial neurolysis was greater than with fascial release of the nerve but was not statistically different from that of epineurial release. At 5 months post crush injury, recovery of crushed nerves that underwent neurolysis was 90% and significantly less at 70.5% in rats not treated with neurolysis, according to CMAP analysis. Two conclusions can be made from this study. First, intraoperative neurophysiologic studies can monitor the immediate results of neurolysis and predict long-term results in the injured nerve. Second, epineurotomy is important in neurolysis, improves the function of the nerve, less invasive, and a slightly more effective technique than perineurotomy.
Keywords
Axonotmesis . Blood–nerve barrier . Compound muscle action potentials . Crush injuries . Diffusion barrier . Electromyography . Electrophysiological testing . Endoneurium . Epineurium . Epineurotomy . External neurolysis . Fascicle . Internal neurolysis . Mechanical compression . Nerve regeneration . Nerve compression syndromes . Neurolysis . Neurophysiologic testing . Perineurium . Perineurotomy. Peroneal nerve . Peroneal neuropathies . Recovery of function
Introduction
Electrophysiological studies record the electrical activity of motor units during the activation of a target muscle [10, 21]. Neurolysis, a surgical technique, is used to restore impaired or interrupted nerve conduction [1, 12]. External neurolysis describes the freeing of a nerve from a surrounding “constrictor” or “distorter,” such as fibrous fascia or scar and epineurium [3]. Internal neurolysis involves opening the external epineurium and perineurium, thereby exposing bundles of nerve fibers to varying degrees [3].
A nerve is divided into fascicles. Inside the fascicles, surrounding and supporting the nerve fibers, is the endoneurium, a collagenous tissue that lacks elastin. The endoneurium contains continuous type capillaries that are linked with tight junctions, forming a blood–nerve barrier [14]. The fascicles are bound by a perineurium composed of flattened cell processes that also act as a barrier, preventing the diffusion of various molecules, including albumin, into the endoneurium [22, 26]. Varying quantities of perineurium-bound fascicles are enveloped and cushioned by the internal epineurium. The fascicle or groups of fascicles within the internal epineurium are encased by the external epineurium or nerve sheath. The external epineurium, a dense layer of connective tissue, holds all the fascicles together to form a peripheral nerve. Tissue and fascia surround each peripheral nerve [3, 20].
Whereas epineurial and perineurial nerve suturing techniques have been comparatively evaluated [30], little is known about the immediate effects of fascial (external) epineurotomy and perineurotomy on compound muscle action potentials (CMAPs). CMAP testing has been used as one reproducible measurement of clinical functional recovery. Our primary goal was to evaluate whether intraoperative studies would predict the long-term efficacy of neurolysis after nerve injury based on CMAP recovery. Our second goal was to learn if the extent of neurolysis would differentially affect the recovery of nerve function as determined by nerve conduction testing.
Materials and Methods
Animal Groups and Surgery
Thirty Sprague–Dawley male rats (average weight 250– 300 g) were used in this study (Fig. 1). Group A (15 rats) had their left peroneal nerves crushed and they underwent neurolysis at 3 months post crush injury. Group B (15 rats) served as a sham control; their left peroneal nerves were crushed but they did not undergo neurolysis. All right peroneal nerves remained intact and served as normal controls (group C, 30 rat nerves). This study design allowed us to minimize the number of experimental animals. All three groups underwent electrophysiological studies on different time schedules.
The rats were caged separately and had free access to both food and water under standard Medical College of Wisconsin Animal Resource Center guidelines. All procedures followed national animal welfare guidelines.
The animals were prepared for aseptic surgery. They received an intraperitoneal injection of sodium pentobarbital (60 mg/ml, 1:10 diluted by saline, 1 cc/100 g body weight) for anesthesia. All of the study’s operations were performed by the same surgeon under a microscope (WILD, Heerbugg, Leitz) with ×36 magnification.
Crushed (left) nerves On the left side of groups A and B, the sciatic, tibial, and peroneal nerves were exposed using a 2-cm incision at the dorsal gluteal area. The peroneal nerve was crushed at a distance of 3 mm from where it branched off the sciatic nerve. This was accomplished using one 5-in. needle holder, 3 mm in width (Weck Stainless, #510321). The needle holders were clicked twice, producing a force of 30 lbs as measured by pressure films that used color intensity to reveal the distribution and magnitude of pressure between two surfaces (Pressurex®, Sensor Products, Madison, NJ, USA). After 1 min of crushing, the nerve was released. CMAPs were measured before and 30 min after the crushing of the nerve. Each test was repeated three times and the maximal response was recorded for each test. In group B, on the left side, crush injury was done but no neurolysis was performed; this was the sham control. In addition to the initial electrophysiological studies performed during the first surgery, further studies were performed at 3 months postoperatively on group A rats and 5 months postoperatively on groups A and B rats. At those times, each rat was reanesthetized and the same surgical site was exposed.
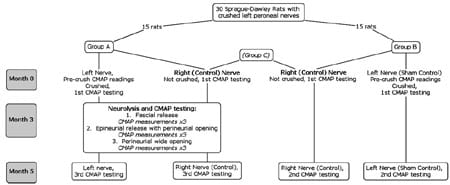
Fig. 1 Study groups and procedures.</st rong>
Control (right) nerves On the right nerves of groups A and B rats, no neurolysis was performed but CMAP tests were carried out immediately after the initial surgery and 5 months later. Group A rats also had CMAP testing at month 3. The right nerves of groups A and B rats were designated as group C.
Electrophysiological Evaluation
We chose CMAP measurement in this study because CMAP reflects the number of excitable motor axons that are responsive to electrical stimulation. It is a reproducible measurement of clinical value. All conduction studies were performed using the same Nicolet Viking EMG machine. The stimulating electrodes were bipolar Teflon-coated stainless steel electrodes separated by 1 mm. The stimulation sites were on the left and right sciatic nerves proximal to the branching point. At each stimulated site, the cathode was distal and the anode was proximal. The ground electrode was a Teflon-coated stainless steel needle inserted into the rat’s tail.
CMAPs were recorded by two monopolar stainless steel needles inserted 3 mm apart in the belly of the extensor digitorum longus muscle. The stimulus was a square electric pulse with a voltage of 19 mV, duration of 0.01 ms, and a frequency of 1 Hz. During these procedures, the room temperature was controlled at 25°C, and a heating pad maintained rat body temperature at 38°C as measured by a rectal temperature probe.
Three months post crush injury Neurolysis of the peroneal nerve was performed in group A rats at 3 months post crush injury and CMAP measurements were recorded after each step of neurolysis. The operation was done using the operating microscope at ×36 magnification. The neurolysis steps were as follows:
- Step 1. The nerve was released from the fascia, and the nerve’s epineurium remained intact.
- Step 1. The nerve was released from the fascia, and the nerve’s epineurium remained intact. direction for a total distance of 13 mm. The perineurium was then incised longitudinally without being opened widely.
- Step 3. After having the longitudinal epineurotomy in step 2, the perineurium was widely opened.
The electrophysiological testing was repeated three times after each step. Thus, there were nine test trials on each side. During the tests, the maximal CMAP amplitude was recorded for both the crushed nerve and the control (right) nerve.
Five months post crush injury The nerves on both the right and left sides of the rats in groups A and B were exposed. The final electrophysiological studies were performed. For rats in group A, it was the third set of CMAP measurements on the exposed nerve and the second set of CMAP measurements on the control (right) nerve. For rats in groups B, it was the second set of CMAP measurements on both the exposed (sham control) nerve and the control (right) nerve. During the tests, the maximal CMAP was recorded for both the crushed nerves and the control and sham control nerves. The animals were sacrificed at the end of this 5-month study.
Results
Before the crush injury, the exposed left peroneal nerves in group A rats had a mean CMAP amplitude of 18.28± 3.50 mV, group B rats had a mean CMAP amplitude of 18.36±0.73 mV, and group C nerves had a mean CMAP amplitude of 18.22±0.54 mV (Table 1). These values were used as self-controls during the first nerve exposure. In comparing these groups, there is no statistically significant difference between the initial CMAP amplitudes (ANOVA test, P>0.5). Thirty minutes after being crushed, the left nerves of rats in both groups A and B had a mean CMAP amplitude of 0 mV, indicating severe crush injury.
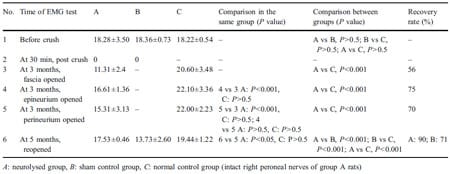
Table 1 Results of electrophysiological studies.
Three months after the crushing of the nerves in group A rats, neurolysis was performed and immediate CMAP analysis was done (step 1). Group B rats were not treated with neurolysis, as they were the sham control group. In group A rats, the mean CMAP amplitude immediately after the release of the nerve from the surrounding fascia was 11.3±2.36 mV in their crushed left nerves and was 20.6± 3.48 mV in the (control) noncrushed right nerves.
Opening the epineurium of the crushed nerves (step 2) of group A rats resulted in an increase in the mean CMAP amplitude to 16.61±1.36 mV. The opening of the epineurium in the crushed nerve thus resulted in a statistically significant increase (P< 0.001) in the CMAP from the CMAP that was measured after only fascial release. The mean CMAP amplitude of the group A (control) noncrushed right nerves after epineurial neurolysis (22.1 ± 3.36 mV) was not markedly different from the value that was measured after fascial release (20.6±3.48 mV).
The CMAP amplitude of the group A crushed left nerves after epineurial release plus wide opening of perineurium (step 3) was 15.31±3.13 mV and that of the group A (control) noncrushed right nerves after perineurial neurolysis was 22.0±2.23 mV. The improvement with this perineurial neurolysis in group A crushed left nerves was still statistically significant in comparison to that of the post fascial release CMAP (P<0.001); however, it was not statistically different from the CMAP that was recorded after epineurial release (P>0.5). The mean CMAP amplitude in the group A (control) noncrushed right nerves (22.0± 2.23 mV) was again statistically unaffected by the neurolysis with any differences being insignificant (P>0.5).
The final results of the neurolysis were obtained 2 months later in the group A rats, which was 5 months after the original crush injury. Both the crushed and the uncrushed group A nerves had already been released with perineurial neurolysis. The CMAP of the group A crushed nerves was 17.53±0.46 mV. The group C nerves had a CMAP of 19.44±1.22 mV. Thus, the recovery of the group A crushed nerves was deemed to be 90% as indicated by CMAP analysis. The rats in group B (sham control) whose crushed nerves were not treated with neurolysis had a mean CMAP amplitude at this time of 13.73±2.58 mVon the left crushed side. The percentage of recovery was significantly lower at 70.5% (t test, P<0.001) than those of the group A rats who had undergone neurolysis after crush injury. The percentage of recovery was calculated by dividing the CMAP on the crushed side by that of the CMAP of the group C nerves.
Discussion
Clinically, neurophysiologic testing is used to evaluate clinical findings and monitor the progression of nerve lesions and reinnervation of muscle [5, 17]. The absence of recovery from a neural lesion as indicated by clinical symptoms and neurophysiologic testing can often lead to surgical exploration and neurolysis [12, 15, 16, 28]. Common intraoperative uses of electrophysiological testing, collectively described by Slimp [25], include monitoring the approach and manipulation of nerves [25], guiding dissection in distinguishing between neural and nonneural tissue [10, 25], identifying and localizing nerves [25], and assessing the nerve’s motor function [25].
During nerve conduction studies, action potentials travel through the nerve and across the neuromuscular junction after the stimulus intensity reaches a threshold value [23]. Measured in millivolts, the summated electrical potential produced is called a CMAP; it provides an indirect but fairly accurate estimate of the number of motor units in the innervated muscles [3, 10, 23]. The CMAP can be measured using needle electrodes inserted in the muscle to derive an estimate of the number of functioning axons that contribute to the electrical potential [3].
It is still difficult to quantify the degree of damage to nerve motor function. Clinically, most ne
urophysiologists believe that the CMAP objectively reflects motor function. For example, a CMAP decrease by 50% often indicates muscular force less than grade 3, as CMAP indicates the relative numbers of excitable motor axons and their attached muscular fibers. In a study by Chang and Lien [7] that compared healthy subjects and subjects with radiculopathy, differences of more than 9.6% in the amplitude of the negative phase CMAP were considered abnormal. CMAP has been used as a measurement for the degree of clinical functional recovery; therefore, we chose to use CMAP in this study. Cooling of the nerve can actually increase the amplitude of the CMAP and, therefore, studies must control for temperature [23]. In our study, the room temperature and rat temperature were strictly controlled and remained constant at all times.
A loss of responsive axons or muscle fibers causes the amplitude of the CMAP to decrease [3, 12, 23]. The amplitude of the distal CMAP drops temporarily in injuries that are purely neurapraxic and recovers unless there is distal disuse atrophy [6]. Crush injuries often result in axonotmesis, which involves axonal damage and Wallerian degeneration [6, 11].
Increased CMAP amplitudes are a sign of muscle reinnervation and recovery of an injured nerve [12, 23]. A study by Hong et al. used CMAP amplitude as an indicator of the recovery rate of crushed nerves in rats after treatments with varying doses of ultrasound thermotherapy [13].
CMAP amplitude has been used to analyze the injury and recovery phases of a nerve. This study uses it to determine the recommended extent of neurolysis. In a 1980 study of chronic ulnar nerve lesions, Nielsen et al. recommended the “abandonment” of interfascicular neurolysis after analyzing parameters that included preoperative and postoperative EMG readings [21]. In their nine patients, the target of the neurolysis was epineurial and perineurial fibrosis [21]. They concluded that the technique posed a great risk of further damage to the nerve, as exhibited by the deterioration of electrophysiological measurements that were normal or near normal before the neurolysis [2, 21]. The major difference between the study by Nielsen et al. and our own is that their nerve testing was not used intraoperatively. Therefore, they did not analyze the immediate effects of neurolysis or measure the different readings that resulted from dissecting through each neural layer.
Damage to the blood vessels of peripheral nerves has been shown to result in the exudation of albumin into the endoneurium. The resulting protein-rich fluid collection can create increased scar and pressure within the endoneurium [18, 19]. The perineurium normally prevents this diffusion of albumin [22, 26]. Widely opening the perineurium intraoperatively damages this barrier. This could account for the post neurolysis deterioration of electrophysiological measurements in the study by Nielsen et al. [21], as well as the lack of a statistically significant improvement between wide longitudinal epineurotomy versus perineurotomy in our own study.
One might ask how improvement of conduction could occur so rapidly. CMAP amplitude measures the number of stimulated and responsive axons. Axonal growth does not occur quickly enough to be the mechanism. As mechanical compression results in intraneural edema [27] by increasing the permeability of endoneurial capillaries [14], relief of the external compression reduces nerve edema and endoneurial pressure [14]. This subsequently improves blood flow, improves Na+/K+ATPase function and allows the action potentials to regenerate. External mechanical compression also leads to myelin deformity and distortion of the internodal sodium channels. Mechanical release of compression would result in the ability to record responses from certain myelinated fibers that were not responsive before.
Whereas the Nielsen et al. study involved human ulnar nerve palsies [21], this study involved peroneal nerve crush injuries in rats. Peroneal neuropathies in humans occur frequently because of the vulnerable position of the nerve near the fibular head [3]. Various etiologies of peroneal neuropathies include anterior compartment syndrome [3], squatting [4, 24, 29], and leg crossing [4, 9, 15, 24] among other causes [8, 9].
As to how the CMAP correlates with clinical recovery, our study did not evaluate functional recovery in rodents. Axonal recovery was the focus of our study and there is agreement among many investigators that the correlation between axonal recovery and electrophysiological measurements is very good. Future animal studies should address if the CMAP results of this study would be paralleled in muscle tetany force measurements or gait analysis. As the recovery of nerves has been shown to take between 12 and 30 months on average [15], studies that involve a longer follow-up would be most accurate.
Conclusions
First, intraoperative neurophysiologic studies can monitor the immediate results of neurolysis and predict long-term results in the injured nerve based on CMAP recovery. Second, epineurotomy is important in neurolysis and improves nerve function as demonstrated by using CMAP amplitude. Results of wide longitudinal epineurotomy are similar to those of perineurotomy. We recommend epineurotomy as a less invasive and slightly more effective technique for the treatment of crushed nerves.
Acknowledgment
The authors wish to thank Beth Kaczmarek, Editorial Consultant in the Department of Plastic Surgery, Medical College of Wisconsin, for her assistance with manuscript revision.
References
- Babcock WW. A standard technique for operations on peripheral nerves with especial reference to the closure of large gaps. Surg Gynecol Obstet 1927;45:364–78.
- Barrios C, Ganoza C, de Pablos J, et al. Posttraumatic ulnar neuropathy versus non-traumatic cubital tunnel syndrome: clinical features and response to surgery. Acta Neurochir (Wien) 1991;110:44–8.
- Birch R, Bonney GWL, Wynn Parry CB. Surgical disorders of the peripheral nerves. New York: Churchill Livingstone; 1998, p. 539.
- Brown WF, Watson BV. Quantitation of axon loss and conduction block in peroneal nerve palsies. Muscle Nerve 1991;14:237–44.
- Cameron MG, Stewart OJ. Ulnar nerve injury associated with anaesthesia. Can Anaesth Soc J 1975;22:253–64.
- Carter GT, Robinson LR, Chang VH, et al. Electrodiagnostic evaluation of traumatic nerve injuries. Hand Clin 2000;16:1–12.
- Chang CW, Lien IN. Spinal nerve stimulation in the diagnosis of lumbosacral radiculopathy. Am J Phys Med Rehabil 1990;69:318–22.
- Cruz Martinez A. Slimmer’s paralysis: electrophysiological evidence of compressive lesion. Eur Neurol 1987;26:189–92.
- de Carvalho M, Miguel S, Bentes C. Sensory potential can be preserved in severe common peroneal neuropathy. Electromyogr Clin Neurophysiol 2000;40:61–3.
- Deletis V, Morota N, Abbott IR. Electrodiagnosis in the management of brachial plexus surgery. Hand Clin 1995;11:555–61.
- Diao E, Vannuyen T. Techniques for primary nerve repair. Hand Clin 2000;16:53–66.
- Goitz RJ, Tomaino MM. Management of peroneal nerve injuries associated with knee dislocations. Am J Orthop 2003;32:14–6.
- Hong CZ, Liu HH, Yu J. Ultrasound thermotherapy effect on the recovery of nerve conduction in experimental compression neuropathy. Arch Phys Med Rehabil 1988;69:410–4.
- Kobayashi S, Meir A, Baba H, et al. Imaging of intraneural edema by using gadolinium-enhanced MR imaging: experimental compression injury. Am J Neuroradiol 2005;26:973–80.
- Kim DH, Kline DG. Management and results of peroneal nerve lesions. Neurosurgery 1996;39:312–9.
- Kim DH, Murovic JA, Tiel RL, et al. Management and outcomes
in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery 2004;54:1421–8. - Kline DG. Physiological and clinical factors contributing to the timing of nerve repair. Clin Neurosurg 1977;24:425–55.
- Low PA, Dyck PJ, Schmelzer JD. Chronic elevation of endoneurial fluid pressure is associated with low-grade fiber pathology. Muscle Nerve 1982;5:162–65.
- Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome”. J Neurol Neurosurg Psychiatry 1983;46:1119–24.
- Matloub HS, Yousif NJ. Peripheral nerve anatomy and innervation pattern. Hand Clin 1992;8:201–14.
- Nielsen VK, Osgaard O, Trojaborg W. Interfascicular neurolysis in chronic ulnar nerve lesions at the elbow: an electrophysiological study. J Neurol Neurosurg Psychiatry 1980;43:272–80.
- Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol 1966;7:1–15.
- Peterson GW, Will AD. Newer electrodiagnostic techniques in peripheral nerve injuries. Orthop Clin North Am 1988;19:13–25.
- Singh N, Behse F, Buchthal F. Electrophysical study of peroneal palsy. J Neurol Neurosurg Psychiatry 1974;37:1202–13.
- Slimp JC. Intraoperative monitoring of nerve repairs. Hand Clin 2000;16:25–36.
- Soderfeldt B, Olsson Y, Kristensson K. The perineurium as a diffusion barrier to protein tracers in human peripheral nerve. Acta Neuropathol 1973;25:120–6.
- Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 1976;39:615–26.
- Thoma A, Fawcett S, Ginty M, et al. Decompression of the common peroneal nerve: experience with 20 consecutive cases. Plast Reconstr Surg 2001;107:1183–9.
- Togrol E. Bilateral peroneal nerve palsy induced by prolonged squatting. Mil Med 2000;165:240–2.
- Tupper JW, Crick JC, Matteck LR. Fascicular nerve repairs: a comparative study of epineurial and fascicular (perineurial) techniques. Orthop Clin North Am 1988;19:57–69.