B. M. Daubs, R. M. McLaughlin, E. Silverman, J. Rizon
Department of Clinical Sciences, College of Veterinary Medicine, Mississippi State University,
Mississippi, USA
Summary
created in each of the 52 humeri collected from 26 dogs. One humerus from each pair was stabilized with a 2.0 mm cortical bone screw which was inserted in lag fashion. The other humerus from each pair was stabilized with a 2.2 mm threaded diameter Orthofix pin inserted across the condyle. Prior to each repair, an antirotational K-wire was placed and then the Pressurex® Sensitive film was inserted in the osteotomy site in order to determine the compressive pressure (MPa), compressive force (KN), and area of compression (cm2) achieved during fixation. The maximum insertional torque achieved before stripping was measured for each implant. The mean compression generated by insertion of a 2.0 mm lag screw was 20.36 ± 1.51 MPa compared to 18.88 ± 1.76 MPa generated by a 2.2 mm Orthofix pin (p < 0.003). The mean area of compression generated by insertion of a 2.0 mm lag screw was 2.39 ± 1.29 cm2, compared to 1.16 ± 0.84 cm2 generated by insertion of a 2.2 mm Orthofix pin (p < 0.0001). The mean compressive force (compression x area compressed) generated by insertion of a 2.0 mm lag screw was 4.96 ± 2.90 Kn, compared to 2.20 ± 1.65 Kn generated by insertion of a 2.2 mm Orthofix pin (p < 0.0001). The mean insertion torque to failure for the lag screws was 0.49 ± 0.07 NM, compared to 0.91 NM ± 0.18 NM generated by the Orthofix pins (P < 0.0001). Both repair methods are likely to be acceptable for the repair of similar fractures in small breed dogs.
Keywords
Humeral condylar fracture, Orthofix pin, compression
Introduction
Distal humeral fractures are common in dogs and often occur as a result of a minor trauma, such as playing, running, and falling (1–5). Fractures which involve the capitulum (lateral aspect of the condyle) are most common (56–67%), while dicondylar fractures (35%) and trochlear fractures occur less frequently (4%-11%) (1–5). The majority of fractures which involve the lateral portion of the humeral condyle occur in animals less than one year of age, and are often associated with incomplete ossification of the humeral condyle (1, 4, 6–10). Spaniel dogs are over represented, especially Cocker Spaniels and Springer Spaniels (1, 4, 6–8). The majority of distal humeral fractures (63%) involve the articular surface (1). Anatomic reduction and rigid internal fixation are required to restore joint function and minimize the development of osteoarthritis (3, 4, 11–15). The most common method which has been described for the repair of distal humeral condylar fractures is the achievement of interfragmentary compression by inserting a transcondylar lag screw or a transcondylar lag screw in conjunction with an anti-rotational Kirschner wire (2, 4, 11, 12, 15–18). The insertion of a transcondylar lag screw using a minimally invasive surgical technique has been described (12, 15). Recently, the use of Orthofix self-compressing bone pins was described for the stabilization of distal humeral condylar fractures and was reported to have been successful in a series of clinical cases (13, 16). Another recent study reported equivalent biomechanical properties when lateral humeral condylar fractures were repaired with either a self-compressing Orthofix pin or a bone screw (2.7 mm and 3.5 mm) placed in lag fashion (19). To date, there there have not been any reports that document the compression generated at the fracture site by lag screw fixation or Orthofix pin fixation when used for stabilization of distal humeral condylar fractures.
Guille et al. reported that 10 out of 23 cases of distal humeral condylar fractures which were repaired with Orthofix pins had a post-operative reduction gap or a step of between 1.0 and 3.0 mm (16). Of those 10 cases, seven reported a satisfactory outcome at follow-up (16). Vida et al. reported that there were minimal ( < 1 mm) or no gaps visible at the articular surface after repair of all of the simulated fractures using both Orthofix pins and lag screw fixation (19). In a closed reduction technique, Cook et al. reported a postoperative malreduction of less than 1.5 mm in five out of 11 fractures. All of the dogs in that study had satisfactory outcomes, however, one dog had an additional surgery after implant failures (12). The relationship between the malreduction and the clinical outcome or development of osteoarthritis was not reported (12). Follow up was available on 10 repairs and the outcome was satisfactory (12). The compression that was achieved using various fracture repair methods has been quantitated using force transducers (20–23). The resultant force measurement has been measured at the screw head and near cortex interface (20–22). The use of the Pressurex® Sensitive filma will allow the compression to be determined at the actual interfragmentary sight.
The purpose of this study is to document the compressive forces that were generated across distal humeral condylar fractures by the insertion of 2.2 mm Orthofix self-compressing bone pins or 2.0 mm bone screws placed in lag fashion. The maximum torque that may be applied before stripping the pin or lag screw will also be determined.
Hypothesis
The compression generated at maximum torque by 2.2 mm Orthofix self-compressing bone pins will be equivalent to the compression generated at maximum torque by a 2.0 mm cortical bone screws placed in lag fashion in simulated lateral humeral condylar fractures.
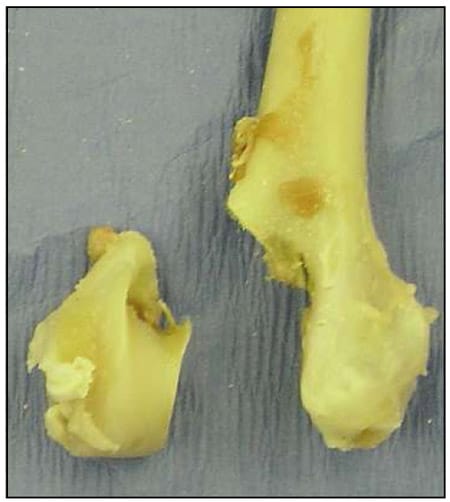
Fig. 1 Lateral condylar fracture model using the right humerus.

Fig. 2 Scanned image of Pressurex® film after compression. Coloured regions indicate areas of compression generated across the fracture surface. The image on the left is the scanned image of the Pressurex® film after compression of the right humeri using the Orthofix pin method. The image on the right is a scanned image of the Pressurex® film after compression of the left humeri using the lag screw method.

Fig. 3 Digitized image of Fig. 2, (Pressurex® film after scanning). Colours indicate the various magnitudes of compression generated across the fracture surface. Red indicates greater compression approaching
or greater than 48.95 Mpa; purple indicates compressing approaching or less than 9.65 MPa. The units on the left are PSI (1400 PSI = 9.68 MPa to 7100PSI = 48.95 MPa). The image on the left is a digitized image of the Pressurex® film after compression of the right humeri using the Orthofix pin method. The image on the right is the digitized image of the Pressurex® film after compression of the left humeri using the lag screw method.
Methods and Material
Twenty-six pairs of canine humeri were collected from dogs (16 female, 10 male) euthanatized for reasons unrelated to this study. The mean weight of the dogs was 13.53 kg ± 5.14 kg and the mean age was 31.92 months ± 33.59 months. All soft tissue was removed and the humeri were wrapped in saline-soaked (0.9%) towels and frozen until the day of testing. Prior to testing, each humeri was thawed at room temperature for 24 hours. The testing was completed over four days and the temperature ranged from 71° to 73.4°. The humidity ranged from 53% to 67%.
The paired humeri were randomly separated into two groups (Group S – Screw stabilization; Group P – Orthofix Magic Pin stabilization). A simulated lateral humeral condylar fracture was created in each bone using a Stryker bone sawb. First, an osteotomy was performed in the sagittal plane originating at the intercondylar groove and terminating at the supratrochlear foramen. A second osteotomy was performed 45 degrees to the humeral shaft originating at the epicondyle and advancing medially, terminating at the supratrochlear foramen (Fig. 1). A 1.2 mm anti-rotational Kirschner wire was then inserted from the lateral epicondyle across the epicondylar osteotomy into the body of the humerus.
After the creation of the simulated fracture, two 2.5 cm x 2.5 cm pieces of Pressurex® Sensitive Film b,c were placed in a plastic sheath (prepared using a whirl pack bagd) and inserted between the capitulum and trochlea of each fracture. Both Low and Medium Pressurex® Film were used to measure a wider range of compression. The fractures in Group S (n= 26) were then stabilized by transcondylar insertion of a 2.0 mm cortical bone screw and washer placed in lag fashione,f,g. The standard technique for lag screw insertion was used. A 2.0 mm glide hole was drilled in the capitulum, and a 1.5 mm thread hole was drilled in the trochlea. The screw was tightened using a dial indicator torque screw driverh to quantify maximum insertion torque (Nm) until the head of the screw stripped. The fractures in Group P (n= 26) were stabilized with a 2.2 mm threaded diameter Orthofix pini,j inserted across the condyle as previously described (13, 16).The pin was tightened using a dial indicator torque driver to quantify maximum insertion torque (Nm) before the bone stripped. The orthofix washer was used on both repair methods to ensure that any difference detected in the compression generated was not associated with a greater surface area contacting the bone at the near cortex.
The implants and Pressurex® film were removed as soon as the screw or pin had been completely tightened. The medium film along with temperature and humidity data was submitted to Sensor Products, Inc.k for analysis. Sensor Products, Inc. used a standard imaging protocol that consisted of scanning the images on a flat bed scannerl, digitizing and analyzing the images using Topaq softwarem. The threshold used was 48.95 MPa, which is the maximum compression that can be detected by the medium Pressurex® film. The compressive pressure (MPa), compressive force (KN) and area of compression (cm2) across the osteotomy site were determined, the compressive pressure was determined by the area compressed on the film and was not based on the simulated fracture interface area (25–27) (Figs. 2 and 3) The data was then imported into Microsoft Exceln and sent back to the investigators. The compressive force was calculated by multiplying the average compression x area compressed. A map reprerepresenting the distribution of compression was also created.
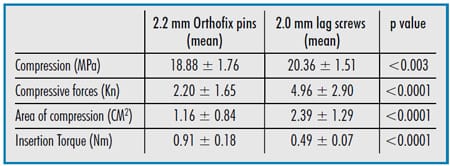
Table Summary of statistically significant results.
Statistical analysis
The compression generated (MPa), compressive force (KN), area of compression (cm2) and maximum insertion torque (Nm) were analyzed using a paired t test at the 5% level of significance. The normality assumption was examined using frequency histograms and normal probability plots; when this assumption appeared to be substantially violated, the data was reanalyzed using the Wilcoxon signed-rank test. Confidence intervals were used to assess the clinical importance of differences between repair methods (28). Statistical analyses was performed using the GLM and UNIVARIATE procedures of the SAS System for Windows, Version 9.1.o P values of < 0.05 were considered statistically significant. All results are reported in mean values ± standard deviation.
Results
The mean compression generated by the insertion of a 2.0 mm lag screw (20.36 ± 1.51 MPa) was significantly greater (p < 0.003) than the mean compression generated by the insertion of a 2.2 mm Orthofix pin (18.88 ± 1.76 MPa) (Table 1).
The mean area of compression generated by the insertion of a 2.0 mm lag screw (2.39 ± 1.29 cm2) was significantly greater (p< 0.0001) than the mean area of compression generated by insertion of a 2.2 mm Orthofix pin (1.16 ± 0.84 cm2).
The mean compressive force (compression x area compressed) generated by the insertion of a 2.0 mm lag screw (4.96 ± 2.90 Kn) was significantly greater (p< 0.0001) than the mean compressive force generated by the insertion of a 2.2 mm Orthofix pin (2.20 ± 1.65 Kn).
The lag screws and Orthofix pins were inserted to failure. All of the screws failed by disruption of the screw head such that further tightening was impossible. The Orthofix pins failed by stripping at the pinbone interface. The mean insertion torque to failure for the lag screws (0.49 ± 0.07 NM) was significantly less (P< 0.0001) than that for Orthofix pins (0.91 NM ± 0.18 NM).
Discussion
This study documented that mean compression, compressive force and area of compression are greater after insertion of 2.0 mm lag screws across a simulated distal humeral condylar fracture then after insertion of 2.2 mm Orthofix pins. Two mm bone screws were evaluated for the following reasons: they were similar in size to the 2.2 mm Orthofix pins, they are clinically used in very small dogs, and because 2.7 mm and 3.5 mm screw have been previously tested (19).
The screws and pins used in this study were inserted to failure in order to determine the maximum torque that could be applied (in order to achieve maximum compression) prior to failure. The 2.0 mm screws have a cuneiform screw head that failed during screw insertion. The authors expected the screws to fail at the screw-bone interface (just as the Orthofix pins did) and failure of the screw heads was not anticipated. As a result, the lag screws were not inserted until failure had occurred at the screw-bone interface, which most likely would have increased the compression generated across the fracture line. However, the compression generated with the lag screws was still significantly greater than that achieved with the Orthofix pins. The use of 2.7 or 3.5 screws would probably create greater compression due to the greater thread-bone interface, and the square-drive screw heads would be less likely to fail during insertion.
The anti-rotational K wire was inserted before the lag screw placement, Orthofix pin placement, or placement of pressure film. This order wa
s chosen in order to avoid creation of a compressive force during K-wire insertion and to assist in the reduction of the fracture during insertion of the lag screw and the Orthofix pins. The technique allowed accurate implant placement without the need for bone clamps which may have affected compression measurements. The model use in this study differs from that used by Vita et al. inasmuch as a 1 cm gap was not produced proximal to the supratrochlear foramen. A gap was unnecessary in this study since an axial loading force was not applied and this would have made it difficult to insert the anti-rotational K wires.
The pressure sensitive film used in this study included both Low and Medium Pressurex® Film. Both were initially used to permit a wider range of measurable compression. However, after evaluation of the pressure film, it was apparent that a variety of colour shades was evident on the Medium Pressurex® film (indicating various levels of compression) while only dark shades were visible on the Low Pressurex® film (indicating that compression exceeded the measurable range of 2.41 to 9.65 MPa). For this reason only the Medium Pressurex® film, measuring pressures from 9.65 to 48.95 MPa, was analyzed. Pressures exceeding 48.95 could not be measured using the Medium film; however, the measurements collected in this study fall well within the range detectable by the medium Pressurex® film. The film was covered with plastic during testing since oils from the bone can affect results.
This film measures the one time maximum compression generated when sufficient torque is applied to strip the implants at the screw head or the implant-bone interface. Obviously, the compression generated in a clinical setting would be less since stripping is to be avoided. In addition, these values were generated using bone that was grossly stripped of soft tissue and a smooth interface between the implants the bone was present. Clinically this is more difficult to achieve as the bone surface is obscured with soft tissue. The compression values over the fracture interface area (not reported in this study) would be less than the compression measurements reported herein. Also, maximum compression measured in this study would likely decrease as the bone, tissue, and implant undergo stress relaxation. In this study, the insertion torque required for the Orthofix pin to strip at the bone pin interface (0.91 ± 0.18 Nm) was greater than the insertion torques reported by Vida et al. (0.57 ± 0.07 Nm and 0.73 ± 0.11 Nm) (19). In the study reported here, the pins were inserted to failure at the pin-bone interface, as indicated by a sudden decrease in insertional torque. When placing the pins, insertion torque increased and then plateaued (for 2–3 revolutions) before the torque decreased. The rate of pin insertion may also affect the maximum torque achieved before failure, though the insertion rate was not measured in either of the studies.
Although the length of time required for insertion was not evaluated in this study, subjectively the Orthofix pins took considerably less time to insert. This is consistent with a previous study which indicates that the insertion time into foam blocks for the Orthofix pin (114 seconds) was significantly less (P < 0.05) than the insertion time for threaded cancellous screws (207 seconds) (24).
Compression generated at the fracture surface increases the degree of friction, thus helping to stabilize the fracture by counteracting forces that cause micromotion (29). Shear stress and bending stress would also be reduced the more the degree of compression increases. The degree of compression to enhance healing is unknown, however, distal humeral condylar fractures which have been repaired using the lag screw method in previous studies have had good results (2, 4, 12, 18). Though the difference in mean compression generated by 2.0 mm lag screws (20.36 MPa) and 2.2 mm Orthofix pins (18.88 MPa) was relatively small, the area of compression was significantly greater when a lag screw was used. Compressive force (compression x area compressed) was therefore also greater when a lag screw was inserted. This difference was statistically significant, however, the true clinical significance is unclear. The larger area of compression generated by lag screw insertion may positively affect fracture stability and fracture healing, though these parameters were not evaluated in this study. Subjectively there were not any gaps or steps at the osteotomy site after reduction using either method. The results of this study demonstrate that interfragmentary compression is generated with Orthofix pins. Also, previous studies indicate that Orthofix pin fixation (with an anti-rotational K-wire) is a clinically acceptable method for the repair of lateral humeral condylar fracture (13, 16, 19). Our findings support the conclusions drawn by other authors, namely that Orthofix pins are acceptable for the repair of humeral condylar fractures, particularly in small breed dogs (16, 19).
Acknowledgement
The authors acknowledge Dr. Brian K. Sidaway for his assistance in the design of this study.
References
- Bardet JF, Hohn RB, Rudy RL. Fractures of the humerus in dogs and cats a retrospective study of 130 cases. Vet Surg 1983; 12: 73–77.
- Vannini R, Smeak DD, Olmstead ML. Evaluation of surgical repair of 135 distal humeral fractures in dogs and cats. JAmAnim Hosp Assoc 1988; 24: 531–536.
- Vannini R, Olmstead ML, Smeak DD. An epidemiological study of 151 distal humeral fractures in dogs and cats. JAmAnim Hosp Assoc 1988; 24: 531–536.
- Deny JR. Condylar fractures of the humerus in the dog: a review of 133 cases. J Small Anim Pract 1988; 24: 185–197.
- Rorvik AM. Risk factors for humeral condylar fractures in the dog: A retrospective study. J Small Anim Pract 1993; 34: 277–282.
- Meyer-Lindberg A, Heinen V, Fehr M et al. Incomplete ossification of the humeral condyle as the cause of lameness in dogs. Vet Comp Orthop Traumatol 2002; 3: 187–194.
- Butterworth SJ, Innes JF. Incomplete humeral condylar fractures in the dog. J Small Anim Pract 2001; 42: 394–398.
- Unger M, Montavon PM, Heim UFA. Classification of fractures of the long bones in the dog and cat: Introduction and clinical application. Vet Comp Orthop Traumatol 1990; 3: 41–50.
- Marcellin-Little DJ, DeYoung DJ, Ferris KK et al. Incomplete ossification of the humeral condyle in spaniels. Vet Surg 1994; 23: 475–487.
- Kaderly RE, Lamothe M. Incomplete humeral condylar fracture due to minor trauma in a mature cocker spaniel. J Am Anim Hosp Assoc 1994; 28: 475–487.
- Matthiesen DT, Walter M. Surgical management of distal humeral fractures. Compend Contin Educ Pract Vet 6 1984; 1027–1036.
- Cook JL, Tomlinson FL, Reed AL. Fluoroscopically guided closed reduction and internal fixation of fractures of the lateral portion of the humeral condyle: prospective clinical study of the technique and results in ten dogs. Vet Surg 1999; 28: 315–321.
- Lanz OI, Lewis DD, Newell SM. Stabilization of a physeal fracture using an Orthofix partiallythreaded Kirschner wire. Vet Comp Orthop Traumatol 1999; 12: 88–91.
- Larsen LJ, Roush JK, McLaughlin RM et al. Microangiography of the humeral condyle in cocker spaniel and non-cocker spaniel dogs. Vet Comp Orthop Traumatol 1999; 12: 134–137.
- Herron MR. Lateral condylar fractures of the humerus: a method of closed repair. Canine Practice 1975; Jan-Feb: 30–34.
- Guille AE, Lewis DD, Anderson TP et al. Evaluation of surgical repair of humeral condylar fractures using self-compressing orthofix pins in 23 dogs. Vet Surg 2004; 33: 314–322.
- Elkins AD, Tangner C
H, Herron MR. Lag screw fixation of capitular fractures of the humerus. Calif Vet 1982; 1: 13–16. - Anderson TJ, Carmichael S, Miller A. Intercondylar humeral fracture in the dog: A review of 20 cases. J Small Anim Pract 1992; 31: 437–442.
- Vida JT, Pooya H, Sasseur PB et al. Biomechanical comparison of Orthofix pins and cortical bone screw is a canine humeral condylar fracture model. Vet Surg 2005; 34: 491–498.
- Johnson KA, Smith FW. Axial compression generated by cortical and cancellous lag screws in the distal phalanx. Vet J 2003; 166: 159–163.
- Phillips JH, Rahn BA. Comparison of compression and torque measurements of self-tapping and pretapped screws. Plast Reconstr Surg 1989; 83: 447–458.
- Draper ER, Wallace AL, Strachan RK et al. The design and performance of an experimental external fixation device with load transducers. Med Eng Phys1995; 17: 618–24.
- Galuppo LD, Stover SM, Jensen DG et al. A biomechanical comparison of headless tapered variable pitch and AO cortical bone screws for fixation of a simulated lateral condylar fracture in equine third metacarpal bones. Vet Surg 2001; 30: 332–340.
- Rovinsky D, Haskell A, Liu Q et al. Evaluation of a new method of small fragment fixation in a medial malleolus fracture model. J Orthop Trauma 2000; 14: 420–425.
- Field JR, Hearn TC, Caldwell CB. The influence of screw torque, object radius of curvature, mode of bone plate application and bone plate design on bone-plate interface mechanics. Injury1998; 29: 233–241.
- Field JR, Hearn TC, Caldwell CB. Bone plate fixation: an evaluation of interface contact area and force of the dynamic compression plate (DCP) and the limited contact-dynamic compression plate (LC-DCP) applied to cadaveric bone. J Othop Trauma 1997; 11: 368–373.
- Field JR, Edmonds-Wilson R, Stanley RM. An evaluation of the interface contact profiles in two low contact bone plates. Injury 2004; 35: 551–556.
- Braitman LE. Confidence Intervals Assess both Clinical Significance and Statistical Significance. Annals of Internal Medicine 1991; 114: 515–517.
- Prieur WD, Sumner-Smith G. Interfragmentary compression. In Manual of Internal Fixation in Small Animals. Brinker WO, Hohn RB, Prieur WD (eds). Berlin: Springer-Verlag 1984; 29–30.


