K. C. Nam¹, S. H. Ahn¹, J. H. Cho², D. W. Kim¹ & S. J. Lee²
¹Department of Medical Engineering, College of Medicine;
²Department of Conservative Dentistry, College of Dentistry, Yonsei University, Seoul, Korea
Abstract
Nam KC, Ahn SH, Cho JH, Kim DW, Lee SJ. Reduction of excessive electrical stimulus during electric pulp testing. International Endodontic Journal, 38, 544–549, 2005.
Aim To measure excessive electrical stimulus time during pulp testing via electromyography (EMG) in the anterior belly of the digastric muscle, voice and finger movement, and to determine whether excessive stimulus time could be attenuated by a specially designed automatic circuit breaker on the basis of the EMG signal.
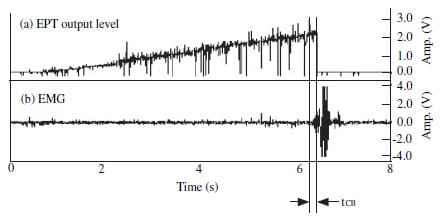
Figure 2 Control of electric pulp tester’s output using EMG response (tCB: time
delay between EMG response start time and EPT circuit break).
Methodology The signals from three human responses (EMG, finger and voice), induced by the DigitestTM (Parkell Inc., Farmingdale, NY, USA) electric pulp tester, were captured using a MP100 (Biopac System Inc., Goleta, CA, USA) and recorded into a personal computer. The excessive stimulus time from activation to the end of electrical stimulation was calculated for each of these three responses. The automatic circuit breaker was designed to disconnect the electrical output of the electric pulp testing (EPT) unit immediately after detecting the preset EMG level (100 mV). Each of the right central incisors and first premolars of 23 healthy individuals (16 males and seven females) was tested to see whether there was a difference in tooth type or gender. This was analysed by Wilcoxon signed rank test (nonparametric paired t-test) and Mann–Whitney test (nonparametric independent t-test), respectively.
Results Amongst three human responses, the electrical onset occurred in the order of EMG, finger and voice. Excessive stimulus time was 347.8 ± 78.3 ms when observed by the EMG, 264.9 ± 63.9 ms when observed by finger span and 229.4 ± 41.8 ms when observed by the voice, which were all found to be significantly different (P < 0.05). When the automatic circuit breaker was used, the excessive stimulus time was 61.0 ms, which was 286.8 ms shorter than that measured from EMG onset when using the conventional EPT.
Conclusions When the automatic circuit breaker was used, excessive stimulus time on the basis of EMG was attenuated on average by 286.8 ms.
Keywords: automatic circuit breaker, electric pulp test, electromyography, excessive stimulus time.
<p>Introduction
Electric pulp testing (EPT) usually elicits a tingling or burning sensation, which can be perceived as discomfort by patients. Although the scan-type EPT ameliorates the patient’s discomfort, to some degree, by narrowing the margin of electrical intensity compared with conventional dial-type EPT, the intensity of the electrical stimulus is designed to increase as the operation time elapses, until the EPT circuit is disconnected. Two pathways exist in order for the circuit to be disconnected after a threshold level stimulation is delivered to the patient. One is the neural pathway for pain perception, which is transferred through the afferent fibres from the pulp to the brain. The other pathway involves voluntary behaviour, that is, the manual switching off of the EPT unit by either the patient or the operator. Some delay, however, is inherent, not only in the neural conduction pathway, but also between the point of the patient’s pain perception and the manual disconnection of the circuit. As the intensity of the EPT stimulus increases proportionally with elapsed time, delayed disconnection of the EPT circuit may result in a considerable amount of unnecessary discomfort. According to experiments with rats, the animal experiences a great deal of stress during the process of EPT. This stress was reported to induce sympathetic nerve stimulation, thereby increasing arterial blood pressure and blood steroid hormone levels (Allen & Pronych 1997, Han et al. 1999).
Several studies regarding the latent time between pulpal stimulation and the electromyography (EMG) response have been undertaken (Matthews et al. 1976, Han et al. 1985, Mason et al. 1986). Matthews et al. (1976) applied a monopolar electrical stimulus of about three times the threshold level to human teeth and observed that the latent time registered on the masseter muscle was 15–20 ms. Similar results in animal experiments were reported by Han et al. (1985) in which the latent EMG responses were found to be 10.83 ms in the mandible and 9.99 ms in the maxilla, when both afferent nerve response and EMG results were monitored simultaneously in cats. However, these experimental results basically only took into account the neural pathway speed from the point of threshold stimulation until the EMG response took place. In order for the patient or the operator to voluntarily disconnect the EPT circuit, more time is inevitable before the EPT circuit can be manually switched off.
The purpose of this study was, therefore, first to measure excessive stimulus time during pulp testing via EMG in the anterior belly of the digastric muscle, voice and finger movement and secondly, to determine whether this excessive stimulus time could be attenuated by a specially designed automatic circuit breaker, on the basis of the EMG signal.
Materials and methods
Recording of human responses from electric pulp stimulation
The electric pulp tester used in this experiment was a DigitestTM (Parkell Inc., Farmingdale, NY, USA) digital scan-type, which increases output intensity as long as the button is in the ‘on’ position. This device has three modes of output: slow, medium and fast, which means that stimulus intensity increases every 400, 200 or 100 ms, respectively. The centre of the facial surface of the tested tooth was dried in order to prevent any electrical contact with the adjacent teeth and then a small amount of toothpaste was applied to establish adequate contact between the tip of the probe and the tooth. In medium mode (200 ms period), three signals generated by EMG, finger movement and voice were collected, until the operator manually disconnected the EPT circuit. The collected signals were recorded into a personal computer using the MP100 system (Biopac System Inc., Goleta, CA, USA). Ea
ch of the right central incisors and first premolars of 23 healthy individuals (16 males of 26.9 ± 2.2 years old and seven females of 25.9 ± 2.9 years old) was tested to see whether there was a difference in tooth type or gender. Only central incisors and first premolars were used to ensure stable access. Informed consent was obtained from the participants after the nature of the procedure and possible discomforts and risks had been fully explained.
EMG response in digastric muscle
The jaw-opening reflex was recorded in the digastric muscle. Two disposable Ag-AgCl surface electrodes (RedDot 3M, St Paul, MN, USA) of 10 mm in diameter were attached onto the skin, over the anterior belly of the digastric muscle. The EMG was in bipolar mode and the distance between the two electrodes was maintained at a consistent 2.5 cm. The ground electrode was attached to the palm of the left hand. The gain of the EMG amplifier (Tel100; Biopac System Inc.) was 10 000·. A 20-Hz high-pass filter was also employed, in order to eliminate any motion artefacts which could possibly be created by the patient’s responses.
Finger response
As soon as the patient perceived a sensation, he or she was instructed to spread out or flex their finger. A bendable resistor sensor (Bend Sensor Flexpoint, Salt Lake City, UT, USA) was attached to the middle finger of the right hand in order to obtain the electrical signal from this finger movement.
Voice response
Voice expression signals, generated by patient response, were captured using a microphone attached to the patient’s neck.
Measurement of excessive stimulus time
Figure 1 demonstrates the electrical output of EPT and other patient’s responses. Figure 1(a) shows the total EPT output level, which increases gradually from the starting point (tS) until the end-point (tE) of the EPT operation. Figure 1(b–d) represent the outputs of EMG, finger and voice responses, respectively. As it is impossible to ascertain at what moment the patient exactly perceived the sensation in this clinical experiment, the earliest onset, which was the EMG response, was regarded as the nearest reference for designating the moment of the patient’s perception. The elapsed time to the end-point of the EPT operation (tE) from the starting point of each response (tEMG, tFS and tV) was also measured and designated as the excessive stimulus time. Statistical analysis was performed using SPSS 8.0 (SPSS Inc., Chicago, IL, USA) with a significant level of P ¼ 0.05. Differences amongst the three patient response times were analysed by Friedman test (nonparametric two-way anova test). Differences in tooth type and gender group were analysed by Wilcoxon signed rank test (nonparametric paired t-test) and Mann–Whitney test (nonparametric independent t-test), respectively.
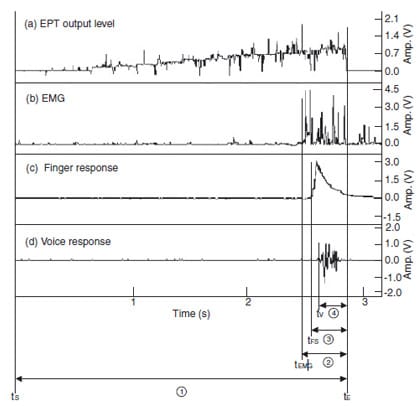
Figure 1 Records of output level, EMG, finger and voice responses during EPT
(tS: start time, tE: end time, tEMG, tFS, tV: EMG, finger
and voice response start time, 1 : total EPT stimulus time, 2 – 4 : measured excessive stimulus
time according to EMG, finger and voice response, respectively).
Automatic circuit breaker
A specially designed switch which could automatically disconnect the EPT circuit was developed, on the basis of the EMG signal. Therefore, as soon as the enveloped EMG signal reached a preset level, which in this case was 100 mV, the switch disconnected the EPT circuit, as shown in Fig. 2. This switch was applied to the DigitestTM and tested on each of the right central incisors and first premolars of 20 healthy individuals (11 males, 26.9 ± 2.2 years old and nine females, 28.2 ± 3.7 years old).
Results
Excessive stimulus time of conventional electric pulp testing
Total excessive stimulus time was calculated from the end of EPT output level (Fig. 1, Table 1) to the start point of each three patient’s responses. The larger the time of the excessive stimulus, the earlier the response occurred, so it was possible to observe that EMG occurred first, followed by finger and voice responses. Each of the three patient response times were significantly different (P < 0.05). However, neither tooth type nor gender significantly affected the response times (P > 0.05).
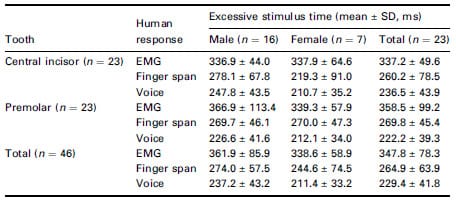
Table 1 Excessive stimulus time measured by EMG, finger span and voice
response, respectively ( 2 , 3 , 4 of Fig. 1)
Excessive stimulus time when using automatic circuit breaker
Table 2 shows the elapsed time from EMG onset until EPT circuit disconnection. On average, the excessive stimulus time was 61.0 ms, which was 286.8 ms shorter than the measurement from EMG onset of conventional EPT use. Neither tooth type nor gender had a significant influence on the response times (P > 0.05).
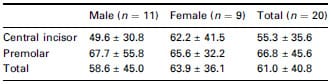
Table 2 Time delay (mean ± SD, ms) between EPT output termination and onset of EMG
Discussion
When three human responses to EPT were compared, the first onset occurred in the EMG, followed by finger and voice responses. Extra stimulus time was defined as the time between the onset point of each patient’s response and the point at which EPT discontinued. In other words, if the EPT circuit could be discontinued as soon as the patient feels electrical stimulation, no extra stimulus time will occur. However, as the patient cannot respond that quickly in a real clinical situation, some latent time between response onset and circuit disconnection inevitably occurs. According to this experiment, the extra stimulus time was 347.8 ± 78.3 ms when observed by the EMG, 264.9 ± 63.9 ms when observed by the finger span and 229.4 ± 41.8 ms when observed by the voice. The mechanism of the scan-type EPT is designed to increase the amount of electrical output per unit time as stimulus time elapses (Fig. 1). In other words, the electrical intensity to which the patient is exposed becomes proportionally greater as the procedure progresses. Therefore, even with a short period of excessive time, the total amount of excessive electrical stimulus would be greater than that of the actual threshold point. Clinically, every effort should be made to reduce unnecessary electrical stimulation above the threshold level. If the exact threshold level for each tooth can be obtained, it would be possible to apply the minimal necessary amount of electrical stimulation to patients. This is, however, impossible, as neither the exact threshold point nor the threshold level can be measured in human subjects. Therefore, substantiation of excessive stimulus time in a clinical situation is a difficult proposition at best. In animal experiments, an electrical stimulation seve
ral times greater than the anticipated threshold level was applied directly to the exposed inferior alveolar nerve sheath, in order to elicit nerve impulse (Koole et al. 1991). The transmission time to the nearby muscle (digastric or masseter) was then measured via EMG signal. Experimentally, the elapsed time between the threshold point and EMG response was reported to be between 10 and 20 ms. Matthews et al. (1976) applied a monopolar electrical stimulus of about three times the threshold level to human teeth, observing that the latent time registered in the masseter muscle was 15–20 ms. Similar results in animal experiments were reported by Han et al. (1985) in which the latent EMG responses were found to be 10.83 ms in the mandible and 9.99 ms in the maxilla, respectively, when both afferent nerve response and EMG result were monitored simultaneously in cats. When this figure (10–20 ms) is interpolated with the excessive stimulus time of EMG (347.8 ms) determined in this study, the total excessive stimulus time would be somewhere around 360 ms (EMG latency time + excessive time measure via EMG).
If the EPT circuit can be shut off immediately after registering EMG, the 347.8 ms of extra time would be saved and that amount of patient discomfort could be alleviated. In order to substantiate this premise, a specially designed switch was developed to automatically disconnect the EPT circuit as soon as the EMG signal occurred. When this automatic circuit breaker was used, the latent time from the EMG onset to the EPT shut-off was measured to be 61.0 ms, which was 286.8 ms (347.8–61.0 ms) less than the values obtained for conventional operation. However, 61.0 ms is still greater than the EMG latency in human experiment (Matthews et al. 1976), which were between 15 and 20 ms. Part of the explanation for this difference may be that, first, there is a circuit operation time delay of about 10 ms during the automatic switching of EPT output. Secondly, the preset value of the EMG signal might have delayed the switch operation, as the switch should trigger only when the enveloped EMG signal reaches the preset value. However, the saving of 286.8 ms is still a substantial improvement.
Whether there was any premature shut-off before the current reached the threshold level was another concern of the present study. The automatic circuit breaker was designed to operate when the EMG output level reached 100 mV. Other signals, lower than 100 mV, were disregarded in order to avoid any electrical noise. All that were tested teeth presented positive responses and it was determined that the threshold levels had been safely reached before the automatic circuit breaker began to operate.
In order to observe muscle response to pulpal irritation, EMG changes from the anterior belly of the digastric muscle, masseter muscle and tongue muscle have been measured (Mahan 1970, Mason et al. 1985, Yu & Park 1999). The anterior belly of the digastric muscle was selected in this experiment not only for the easy placement of the electrodes but also for the fact that the digastric muscle evidenced clear EMG changes when the mouth opened, whilst the masseter muscle exhibited clear EMG changes when the mouth closed (Koole et al. 1991).
Although intramuscular electrodes are employed commonly in animal experiments, they are not practical in human experiments. Koole et al. (1991) compared EMG responses of the masseter, temporalis and anterior digastric muscles obtained by surface and intramuscular electrodes, according to various jaw movements. They observed that surface electrodes gave comparable results in the anterior digastric and masseter muscles. Therefore, two disposable Ag-AgCl surface electrodes (RedDot 3M) of 10 mm in diameter were used over the anterior belly of the digastric muscle.
Conclusion
When an automatic circuit breaker is utilized on the basis of EMG response, excessive stimulus time during EPT was reduced by 286.8 ms. Determination of the exact threshold level and point will require further experimentation.
Acknowledgement
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (02-PJ1-PG10-31401-0003).
References
- Allen GV, Pronych SP (1997) Trigeminal autonomic pathways involved in nociception-induced reflex cardiovascular responses. Brain Research 18, 269–78.
- Han YC, Lee JS, Lee JH (1985) Extracellular recording in trigeminal subnucleus caudalis of the anesthetized cat produced by electrical pulp stimulation. The Korean Journal of Oral Biology 9, 21–9.
- Han SH, Yoon SH, Cho YW, Kim CJ, Min BI (1999) Inhibitory effects of electroacupuncture on stress responses evoked by tooth-pulp stimulation in rats. Physiology and Behavior 66, 217–22.
- Koole P, Jongh HJD, Boering G (1991) A comparative study of electromyograms of the masseter, temporalis, and anterior digastric muscles obtained by surface and intramuscular electrodes: raw-EMG. Cranio 9, 228–40.
- Mahan PE (1970) Jaw depression elicited by tooth pulp stimulation. Experimental Neurology 29, 439–48.
- Mason P, Strassman A, Maciewicz R (1985) Is the jawopening reflex a valid model of pain? Brain Research Reviews 357, 137–46.
- Mason P, Strassman A, Maciewicz R (1986) Intracellular responses of raphe magnus neurons during the jaw-opening reflex evoked by tooth pulp stimulation. Brain Research 379, 232–41.
- Matthews B, Baxter J, Watts S (1976) Sensory and reflex responses to tooth pulp stimulation in man. Brain Research 113, 83–94.
- Yu MK, Park SJ (1999) Changes in autonomic responses and jaw muscle activity induced by tooth pulp stimulation in the rat. Journal of the Korean Academy of Conservative Dentistry 24, 657–65.