Woo-kum Leea, Chien-Hsien Hoa,1, J.W. Van Zeea,*, Mahesh Murthyb
aDepartment of Chemical Engineering, UniÍersity of South Carolina, Columbia, SC 29208, USA
bW.L. Gore & Associates, 402 VieÍe’s Way, Elkton, MD 21922-2200, USA
Abstract
Changes in the performance of a PEM fuel cell are presented as a function of the compression pressure resulting from torque on the bolts that clamp the fuel cell. Three types of gas diffusion layers were studied at 202 kPa and 353 degrees K. An optimum bolt torque was observed when ELAT® or a combination of CARBEL® and TORAYTM gas diffusion media were used as diffusion layers. The optimum is related to the gasket thickness and the measured compression pressure on the diffusion layer. The performance changes may also be related to changes in the porosity, the electrical contact resistance, and the excluded water at the membrane. © 1999 Elsevier Science S.A. All rights reserved.
Keywords: PEM fuel cell; Clamp pressure; Gas diffusion layers
Introduction
Fig. 1 shows a schematic of a typical proton exchange membrane (PEM). fuel cell used in the laboratory for the testing of membrane and electrode assemblies (MEAs). The cell consists of six major parts: end plates, current collectors, graphite flow channel blocks, gaskets, gas diffu- sion layers, and a membrane electrode assembly (MEA). Also shown in Fig. 1 is pressure sensitive film that was used to measure the internal pressure after the six parts are assembled together with four bolts connecting the end plates. Note that this film is removed during operation. As Scherer w1x discusses, the properties of the diffusion layer will impact the optimum performance of the catalyst and the electrode. These layers are porous to allow for distribution of the gases to unexposed areas of the flow channel and this distribution allows for complete utilization of the electrode area. The electrical conductivity of these layers may affect the transport of electrons to the current collector from the electrode. The hydrophobicity of these layers may interact with the amount of water available for hydration at the membrane. These diffusion layers can be porous carbon cloths coated with PTFE as presented by Bevers et al. [2]. Also, these porous cloths or carbon papers may be impregnated with catalyst to form an integrated electrode [3] and these are known as gas diffusion electrodes.
In this paper we study the effect of the compression that may result when a test cell is assembled. That is, the thickness and the porosity of commercially available gas diffusion layers can change depending on the thickness of gasket and the degree to which the gasket is compressed. The thickness may also be affected by the amount of torque exerted on the four bolts as the cell is clamped together. One might expect that a lower porosity would increase the gas phase mass transfer resistance. On the other hand, higher bolt torque may increase the overall electron conductivity of the gas diffusion layer, improve the contact resistance, and hence, minimize some of the electrical resistance losses inside fuel cell. One further aspect of compression is that for a given layer, the compression may change the holdup of water at the electrodes and hence the electrochemical reaction area. As discussed below, these effects may combine to yield an optimum bolt torque for each diffusion layer.
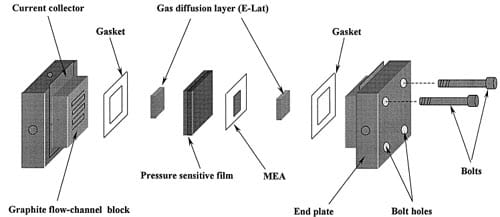
Fig. 1. Schematic of PEM fuel cell showing the location of the components and the pressure sensitive film.
2. Experimental
The experimental studies focused on the performance of PEM fuel cells with different gas diffusion media and with different torque on the bolts that clamp the fuel cell together. All of the data reported here was obtained with a PRIMEAw Series 5000 MEA coated with catalyst (0.3 mg/cm2 Pt loading, 30 mm nominal thickness) by W.L. Gore & Associates. The active area of the membrane was 10 cm2 and the serpentine flow field consisted of 20 equally spaced channels and bends for a total length of approximately 0.6 m between the entrance and the exit. Three types of commercially available gas diffusion layers each with a different thickness were studied: TORAYTM (8 mils = 0.203 x 10-3 m) [4] ELAT® (20 mils = 0.508 x 10-3 m) [5] and CARBEL® Series 100 gas diffusion media [6] combined with TORAYTM (11 mils = 0.279 x 10-3 m). The thickness was measured at nine points on the 10 cm2 surface. An incompressible material with a measured thickness of 7 mils (0.178 x 10-3 m) was used as the gasket for all three layers. The four bolts were threaded into tapped holes on one of the end plates and the cell was compressed by applying a torque on each bolt of either 100, 125, or 150 in. lb . Even though there was a f significant difference between the thickness of the gasket and the thickness of the gas diffusion layer, all of the data reported below corresponds to cells that were free of external leaks.
The experiments consisted of two parts. First, the performance was measured for various bolt torque and diffusion layers. The fuel cells were tested on a test station made by Fuel Cell Technology (Los Alamos, NM). High purity hydrogen (99.997%) and compressed air were used as the fuel and reactant. The flow rates were set according to the measured current at either a high or a low stoichiometry. The high stoichiometry corresponded to flow rates that were 2.0 times greater than that required (by the measured current) for hydrogen (i.e., 100% excess hydro- gen) and 3.5 times greater than that required for air. At low stoichiometry the flow rates were 1.2 and 2.0 times that required for hydrogen and air respectively. Two mass-flow controllers that were calibrated by using a bubble flow meter controlled the gas flow rates. The gas temperature and the degree of humidity were controlled by sparging the gases through water filled tanks held at a temperature of 358°K for the anode and 348°K for cathode. The temperature of the cell was 353°K. The same operating temperatures were used for all experiments and temperature controllers were used to keep these as constants during experiments. Also, the pressures of the anode and cathode sides were both 202 kPa (15 psig). All the tests were performed initially at a constant cell voltage (0.6 V) and the cell currents were recorded by using the related computer software. All of the fuel cells
were held at 0.6 V for 20 h to condition the MEA before the polarization data were obtained. Thus the data presented below correspond to the beginning of life (BOL) of the MEAs. For a polarization curve the cell voltage was changed from 0.3 V to 0.9 V and the resulting currents were recorded. The flow rates were adjusted manually in an iterative manner to maintain a fixed stoichiometry at each cell voltage for the polarization data.
A discussion of the ability to control humidity is relevant to the data presented below. Calibration measurements [7] indicated that sparging the gases through our water-filled tanks provided a controlled temperature but not a constant relative humidity. Although our system did not allow for a check at 202 kPa, we calculated the relative humidity at 101 kPa from gravimetric measurements of condensed water collected by chilling the gases. Our data indicate that sparging the gases through water filled tanks yielded a relative humidity approaching 100% for all of the air flow rates used here. However, for hydrogen the relative humidity varied with flow rate. We use these data to estimate that the relative humidity was 70% ± 5% for hydrogen flow rates corresponding to current densities between 0.2 and 0.6 A/cm2 at 202 kPa and 358°K. For the same temperature and pressure, and for flows corresponding to current densities from 0.8 to 1.2 A/cm2, we estimate that the relative humidity changed from 85% to 95% on the anode side.
After finishing the fuel cell performance tests, the compression pressure inside the fuel cell was measured for different bolt torque and gas diffusion layers. The measurements were performed with the use of a pressure indicating film (PRESSUREXTM, Sensor Products, NJ). In these experiments, ‘Super Low’ and ‘Low’ films were used for the various pressure ranges. The pressure ranges of the Super Low and Low films are 70–350 lbf/in.2 and f 350–1400 lb lbf/in.2, respectively. A swatch of film was prepared with dimensions of 0.05 m by 0.05 m and it was inserted between the MEA and the diffusion layer as shown in Fig. 1. Then, a torque was applied gradually on each bolt of the fuel cell for 120 s. As the pressure was applied, microcapsules on the film were broken and a color-forming material was released and absorbed on the film. The color (red) intensity of the film is directly related to the amount of applied pressure. The greater the pressure, the more intense the color. After waiting another 120 s the fuel cell was opened, and the pressure distribution was observable by the color (red) density pattern formed on the film. The pressure was measured by scanning the color density of the film with a densitometer (FDP-301) supplied by Sensor Products.
3. Results and Discussions
Fig. 2 shows an example of the polarization data at 125 in. lbf bolt torque when either the ELAT® medium or the CARBEL® Series100 gas diffusion medium combined with TORAYe medium was used as a gas diffusion layer. Fig. 2 shows reasonably that the performances are higher for the high stoichiometric flows. At a cell voltage of 0.6 V, the current density dropped from 1.01 to 0.80 A/cm2 for the ELAT® and from 0.8 to 0.5 A/cm2 for the CARBEL®-TORAY® when the flow rates were decreased from the high flow rates to the low flow rates. The differences between the types of diffusion layers are discussed below. We choose to present data below for the low stoichiometry conditions because the mass transfer effects should be more pronounced for these conditions.
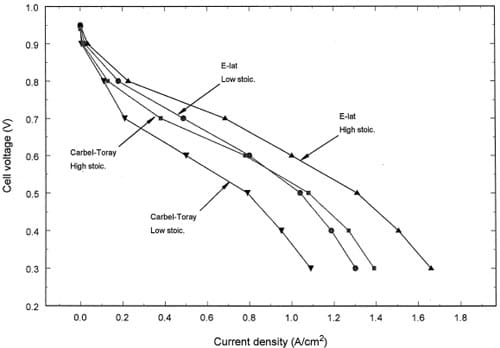
Fig. 2. Effect of stoichiometric (stoich.) flow rate and gas diffusion layer on the cell polarization for a MEA under 125 in. lbf/bolt torque.
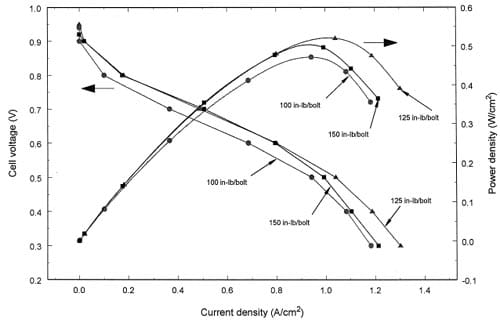
Fig. 3. Effect of torque on the cell polarization and power density at low stoichiometry for a MEA with an ELAT® gas diffusion layer.
Fig. 3 shows the effect of bolt torque on a MEA at low stoichiometric flows. The power density is simply the product of the current and the cell voltage at each current but it reveals the optimum torque and current density at these conditions. Note that we did not try to find the optimum humidity and cell temperature for each MEA and torque at the given stoichiometry of the flow rates. Fig. 3 shows the performance with 125 in. lbf/bolt torque is better than with 100 and 150 in. lbf/bolt when the current density is greater than 0.80 Arcm2. It is interesting to note that the ELAT® suffers a reduction in thickness of greater than a factor of two with the incompressible gasket used here. Since the ELAT® gas diffusion layer is a porous and compressible material, its porosity will decrease and the electrical conductivity will increase as the bolt torque increases. For these two effects, Fig. 3 indicates that compression to 125 in. lbf/bolt enhances the electrical contact but further compression to 150 in. lb rbolt causes f mass transfer resistance. The agreement between the polarization curves until 0.8 A/cm2 indicates that the compression from 125 to 150 in. lbf/bolt does not change the reaction area. At this time we cannot determine if the mass transfer resistance is a result of local H2 or O2 concentration polarization or if the pores are filling with water (i.e., local flooding).
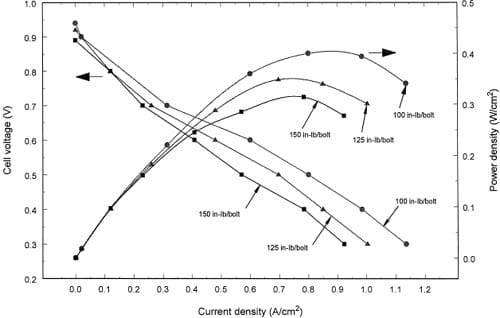
Fig. 4. Effect of torque on the cell polarization and power density at low stoichiometry for a MEA with a TORAYTM gas diffusion layer.
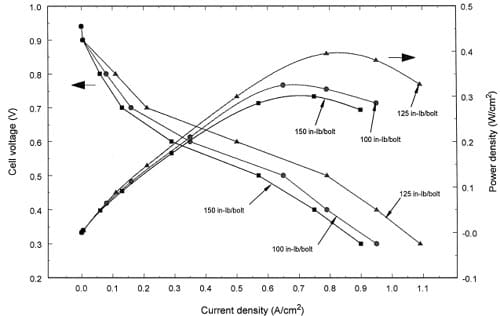
Fig. 5. Effect of torque on the cell polarization and power density at low stoichiometry for a MEA with a combination of TORAYTM and CARBEL® gas diffusion layers.
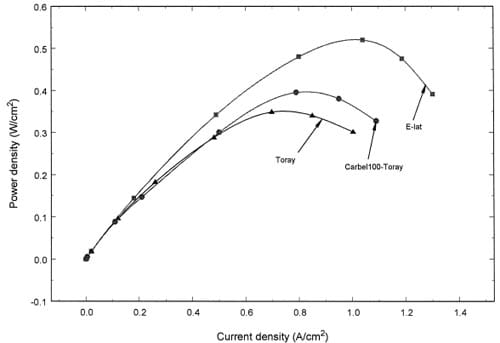
Fig. 6. Comparison of power densities for three diffusion layers at 125 in. lbf/bolt torque and low stoichiometry
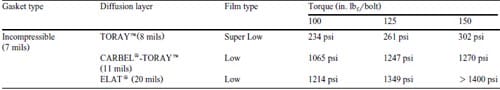
Table 1 – Pressure inside fuel cell as measured by pressure sensitive film. Reported in the common units; psi = lbf/in.2, 1 mil = 2.54 x 10-5 m
Fig. 4 shows the polarization and power density results for a brittle material, TORAYTM, as the gas diffusion layer. Here the trend is that the performance decreases as the bolt torque increases. At 0.6 V cell potential, the current densities are 0.60, 0.49, and 0.40 Arcm2 for 100, 125, and 150 in. lbf/bolt, respectively. These data should be interpreted with our observations that the higher torques break the TORAYTM. With a breaking of the layer, the electrical conductivity between the MEA and the flow plates could be decreased. It is interesting to note that the maximum power density for these conditions is less in Fig. 4 than in Fig. 3. Again, we did not search for the optimum conditions for each diffusion layer, but rather we were interested in showing that the optimum for a
given layer may be influenced by the amount of torque. One may wish to reduce the torque even less than the 100 in. lbf/bolt, but it cannot be too low without changing the gasket material if leaks are to be avoided.
A softer material, CARBEL® Series 100 gas diffusion medium, was combined with the TORAYTM to form the third type of gas diffusion layer. The CARBEL® gas diffusion medium was placed in contact with the MEA and the TORAYTM was placed in contact with graphite flow channel block. The effect of bolt torque on the polarization and power density is shown in Fig. 5. Unlike the results with TORAYTM, there was an optimal bolt torque for this type of gas diffusion layer. This is similar to the use of ELAT® as a diffusion layer. The flexible property of CARBEL® gas diffusion media seems to prevent damage of the TORAYTM diffusion layer even though the additional 3 mils of thickness creates greater reduction in thickness with the 7 mil gasket. At 0.6 V cell potential, the current densities are 0.35, 0.50, and 0.29 A/cm2 for 100, 125, and 150 in. lbf/bolt respectively. It is interesting to note that there are significant differences between torques even at low current densities. We suspect that the compression results in either water blocking some of the reaction area or that it results in water being excluded from the membrane. The water blocking could result in a concentration polarization even at low current densities. These data did not allow us to distinguish the mechanism.
Fig. 6 summarizes the effect of gas diffusion layer on the power density curves at a constant bolt of torque 125 in. lbf/bolt for the low stoichiometric flow rates. At this torque, maximum power densities of 0.52, 0.40, and 0.35 W/cm2 were observed for ELAT®, CARBEL®– TORAYTM, and TORAYTM diffusion layers, respectively. It should be noted that as shown in Fig. 4, a maximum power density for the TORAYTM occurs with 100 in. lbf/bolt rather than with 125 in. lbf/bolt. Again, these power densities may change with humidification temperature and we do not claim that these densities are the maximum for all operating conditions. Our objective here is to show that internal compression resulting from bolt torque and a gasket thickness has an effect on the maximum power density. It may be important to note that Gore now produces a PRIMEA® 5510 Series [8] that yields higher power densities with pure hydrogen fuels and an improved gas diffusion media such as CARBEL®CL.
The averages of five measurements of internal pressure are listed in Table 1. We estimate the measurements to have a “5% variance. These measurements follow trends expected from a consideration of the differences between the gasket thickness and the diffusion layer thickness. The pressures with the CARBEL®-TORAYTM and ELAT® diffusion layers were much higher than the pressure with TORAYTM at the same torque. When a 150 in. lbf/bolt was applied to the fuel cell with an ELAT® diffusion layer, the corresponding pressure exceeded the maximum (1400 lbf/in2) for a Low type of film. We observed channel impressions upon opening the cells when ELAT® was used. Also, we observed that the TORAYTM was brittle and appeared to be broken when it was used alone at these torques. Note that the pressures for the CARBEL®-TORAYTM and ELAT® diffusion layers may exceed those used in fuel cell stack assemblies. The pressures in those assemblies may be limited to prevent crushing the bipolar plates. The pressures shown in Table 1 are achievable in single cell testing.
4. Conclusions
The effects of changing the gas diffusion layer and bolt torque on the performance of a PEM fuel cell have been investigated at fixed stoichiometric flow rates for the fuel and reactant. These effects were shown for a Gore PRIMEAw Series 5000 MEA to illustrate the general trends that should be applicable with other commercially available MEAs. For the three torques used here, an optimal bolt torque (in terms of power density) was obtained for the ELAT® and the combination of CARBEL® and TORAYTM diffusion layers. This optimum was explained in terms of the changes in the porosity and the electrical contact resistance. For the case of a less compressible and somewhat brittle material (TORAYTM used alone., the results show that higher performance was obtained with less torque indicating that the higher bolt torque may have damaged the diffusion layer. However, when soft material (CARBEL® Series 100 gas diffusion media) was combined with the TORAYTM carbon paper one observed the same tendency as for the compressible carbon cloth (ELAT®).
Finally, the pressure inside the fuel cell was measured for various bolt torques. The difference in thickness between the gasket and the diffusion layer affects the pressure inside the fuel cell. According to the results, both the bolt torque and the gas diffusion layer type are important factors for the performance of PEM fuel cell. Future reports will include designed experiments to study the effects of humidity and compressible gaskets for other MEAs at various bolt torques [7].
Acknowledgements
The authors acknowledge financial support for this work from W.L. Gore & Associates. The MEA samples and the many useful discussions provided by Mr. Jeff Kolde (W.L. Gore & Associates) are also gratefully acknowledged. The Department of Energy through Cooperative Agreement Number DE-FG02-91ER75666 provided some of the graduate student stipends for this work. The authors acknowledge Mr. Kevin Houghens and Mr. John Colbert for their measurement of the internal pressures. They were supported by NSF REU Grant Number EEC 9732345 and the State of South Carolina EPSCoR program.
REFERENCES
- G.G. Scherer, Solid State Ionics 94 (1997). 249–257.
- D. Bevers, R. Rogers, M. von Brake, J. Power Sources 63 (1996. 1993–2001).
- T.R. Ralph, G.A. Hards, J.E. Keating, S.A. Campbell, D.P. Wilkinson, M. Davis, J. St.-Pierre, M.C. Johnson, J. Electrochem. Soc. 144 (1997). 3845–3858.
- Toray Industries, Tokyo 103, Japan.
- E-TEK, Natick, MA, USA.
- W.L. Gore & Associates, Elkton, MD, USA.
- W.-k. Lee, Ph.D. dissertation, Department of Chemical Engineering, University of South Carolina, Columbia, SC, 29208, 1999.
- B. Bahar, C. Cavalca, S. Cleghorn, J. Kolde, D. Lane, M. Muthry, G. Rush, Paper Presented at the Second International Fuel Cell Conference, Yamanashi, Japan, August 1998.