A Fuel Cell is an electrochemical device in which a fuel and an oxidizer react, in the presence of an electrolyte and catalyst, to generate an electric current. In other words, fuel cells convert chemical energy into electrical energy. Fuel cells differ from battery cells in that reactants of fuel cells are external to the cells.
Most fuel cell systems have three basic components:
- Unit cells where the electrochemical reactions occur
- Stacks, where individual cells are connected in series to increase overall stack voltage
- Balance of Plant which includes all components that support the fuel cell operation
Typically, unit cells consist of anode and cathode electrodes, an electrolyte membrane, and bipolar plates for current collection and channeling of reaction products. The seven basic types of fuel cells are: Polymer Electrolyte Membrane (PEM) fuel cells, direct methanol fuel cells, alkaline fuel cells, phosphoric acid fuel cells, molten carbonate fuel cells, solid oxide fuel cells, and regenerative fuel cells.
Figure 1 illustrates the operation of a unit cell in a Polymer Electrolyte Membrane (PEM) fuel cell. The hydrogen gas feed is shown at left. At the anode electrode, which contains a platinum catalyst, the H2 gas is converted to two H+ ions and two electrons. The electrons power an external circuit. The H+ ions diffuse through the PEM. At the cathode electrode, two H+ ions combine with two electrons from the external circuit and react with the oxygen gas to form water, which is channeled from the fuel cell. This type of fuel cell is very environmentally friendly since the only effluent is pure water.
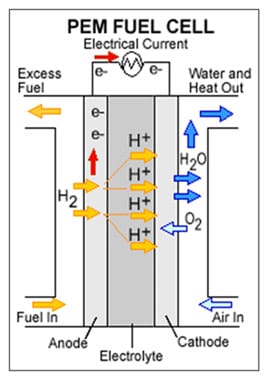
Fig 1: Operation of a PEM Fuel Cell
Since the voltage of a unit cell is only 0.7 to 0.9 V, fuel cells contain stacks of many unit cells in series to maintain sufficient voltage to power electrical equipment. The operating efficiency of the stack depends upon uniform contact pressure between the various plates within the fuel cell. Consequently, fuel cell developers rely on Fujifilm Prescale®, pressure indicating film, to assure uniform pressure between the various elements in the fuel cell stack.
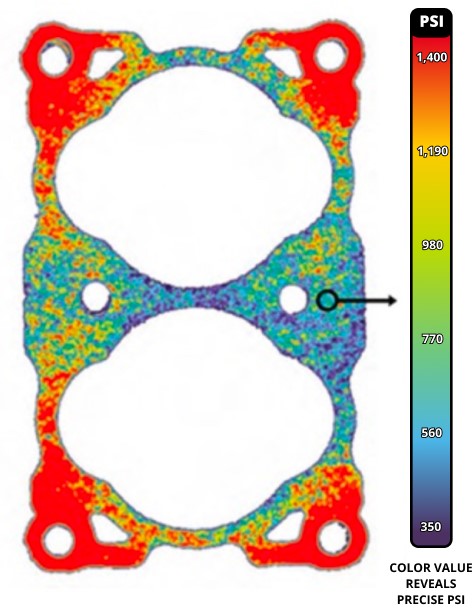
Figures 2–4 illustrate the use of Fujifilm Prescale® in diagnosing pressure non-uniformities within a fuel cell stack. Fujifilm Prescale® reveals both pressure distribution and magnitude between these surfaces in a dramatic surface map. When placed in a fuel cell stack, the film instantaneously and permanently changes color in direct proportion to the actual pressure applied upon it. Pressure magnitude is easily determined by comparing the resultant color intensity to a standardized color correlation chart. Further processing, using Topaq® analysis, dramatizes the pressure differences.
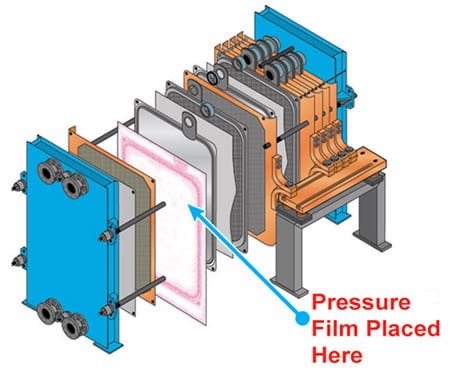 | 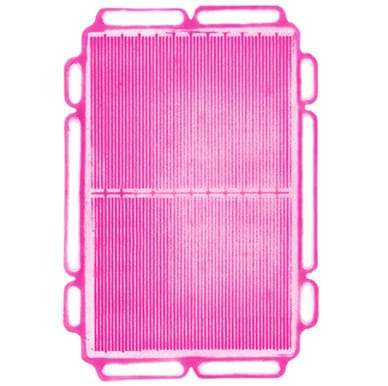 | 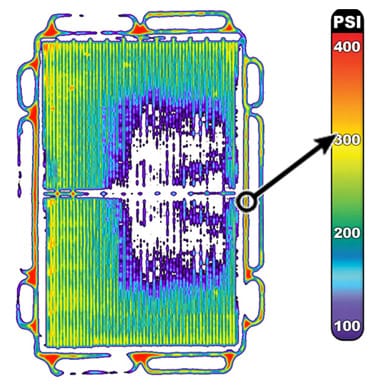 |
| Fig 2: Fujifilm Prescale® Film Placed within Fuel Cell Stack | Fig 3: Fujifilm Prescale® Shows Pressure Profile within Fuel Cell Stack | Fig 4: Fujifilm Prescale® Film after Topaq® Analysis |
Many fuel cell researchers depend upon Fujifilm Prescale® to obtain reliable pressure data to support their studies. For example, Malzbender and Steinbrech published the images in Figure 5 in their article titled, “Advanced Measurement Techniques to Characterize Thermo-Mechanical Aspects of Solid Oxide Fuel Cells”, which was published in the Journal of Power Sources, Vol 173 (2007), pp 60-67.
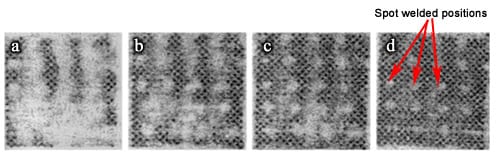
Fig 5: Fujifilm Prescale® images show low pressure areas. Increasing load shows contact with spot welded positions: (a) Pressure = 100 N, (b) 200 N, (c) 400 N, and (d) 600 N.